Chrysis borealis
Figure 179
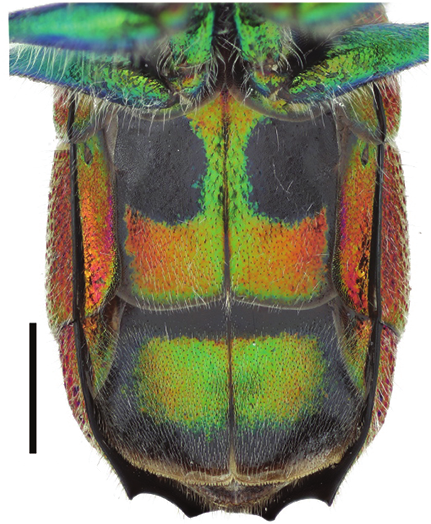
Holotype of Chrysis borealis sp. n. ♀ (HYMNI560) Collected from Ørin, Nord-Trøndelag, Norway. Scale 1 mm.
A recently described species that is extremely difficult to distinguish from Chrysis impressa and Chrysis schencki. Host are Ancistrocerus parietum and probaly also Ancistrocerus scoticus. The species seems to be rare in Fennoscandia. The metasoma is often denser and more strongly punctured than in C. impressa. The mandibel of the female is thicker than in C. schencki.
- Innhold
- Diagnosis
- Description of female
- Description of male
- Geographic variation
- DNA-analysis
- Distribution
- Biology
- Etymology
- Remarks
Diagnosis
Figure 106
Metasoma, dorsal view: C. borealis sp. n. ♀. Scale 1 mm.
Figure 124
Metasoma, ventral view: C. borealis sp. n. ♀. Scale 1 mm.
Figure 133
Metasoma, ventral view: C. borealis sp. n. ♂. Scale 1 mm.
Figure 136
Genital capsule, dorsal view: C. borealis sp. n. ♂. Scale 0.5 mm.
Figure 158
Pronotum and mesoscutum, dorsal view: C. borealis sp. n. ♀. Scale 1 mm.
Length 6–11 mm.
The species is very similar to C. impressa in shape and structure, but the colouration is darker and the length of F1 compared to F2 is larger. The mesoscutum of the female is usually black, violet or dark blue with relatively fine and dense punctation (Figs 158, 179). The punctures are generally of the same colour as the interstices. The metasoma has golden red or reddish tergites (Figs 106, 179) and the sternites are dark green or bluish in the female (Fig. 124), but often with golden red colour in male (Fig. 133). Compared to C. corusca, the body shape is stouter, the metasoma is notably swollen (Figs 106, 179), the black spots of S2 are broader (Figs 124, 133) and the mandible is thinner. Dark specimens of C. schencki are also very similar, but have a thinner mandible and coarser punctation on the scapal basin, and are more slender in habitus. The males of C. borealis in particular are difficult to distinguish from C. impressa and C. schencki. On average, the length ratio F1/F2 is larger (1.3–1.5:1) (Fig. 178), the black spots of S2 are larger (Fig. 133) and the punctation of the mesoscutum is finer in C. borealis. Identification of the males is not always possible with certainty.
Description of female
Body length 7.8–10.3 mm, forewing length 5.1–6.6 mm (n = 12).
Head. Height 1.8–2.1 mm, width 2.3–2.6 mm, length 1.0–1.1 mm, shortest interocular distance 9.4–11.0 mm. Scapal basin green or greenish blue, usually becoming darker blue or violet dorsally below frontal carina. Punctation of scapal basin very dense and fine, partially coriaceous with rugae formed by the puncture margins. Transverse frontal carina well developed, usually relatively evenly arcuate or slightly notched medially. Vertex dark blue, dark violet or black. Pubescence on vertex light brownish. Malar space 1.4 times broader than high. Mandible blackish brown, apically and in inner margin light brown, without subapical tooth. In lateral view, mandible relatively thick (similar to C. impressa), its sides medially almost parallel and basally only slightly concave. Scapus, pedicellus, and F1 with green, blue or violet metallic reflections. Relative lengths of P/F1/F2/F3 are 1/1.8/1.1/0.9. F1 usually 1.4–1.7 times as long as F2. F4–F10 approximately 1.2 times as long as broad.
Mesosoma. Length 3.0–3.8 mm, width anterior to tegulae 2.0–2.7 mm. Length of pronotum medially 0.5–0.6 mm and width at anterior margin 1.8–2.2 mm. Colour of pronotum medially black, dark violet or dark blue, on the margins lighter green, blue or violet, only rarely with golden reflections (Figs 158, 179). Medial groove relatively shallow and indistinctly delimited. Mesoscutum dark violet or black, sometimes with bluish reflections (Figs 158, 179). Punctures generally of the same colour as interstices. Punctation of mesoscutum relatively fine and dense with narrow interstices (Figs 158, 179). Size of punctures on average smaller than in C. impressa and C. schencki. Interstices with scattered small punctures. Tegula green, blue or violet, with paler colour laterally. Mesoscutellum black medially and violet, blue, or sometimes greenish laterally, with irregular large punctures. Metanotum and propodeum violet, blue or greenish. Outer margin of lateral propodeal teeth straight or slightly concave. Pubescence on mesosoma whitish. Legs violet, blue or greenish, but tarsal segments dark brown. Wing venation as in C. impressa and C. schencki.
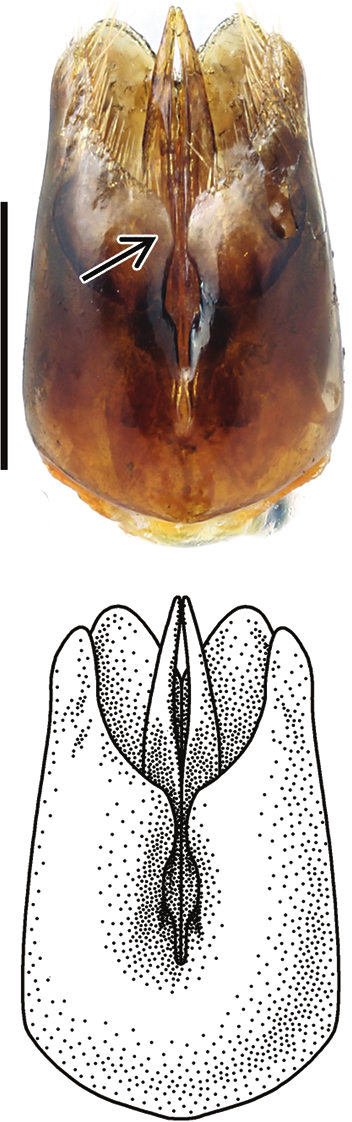
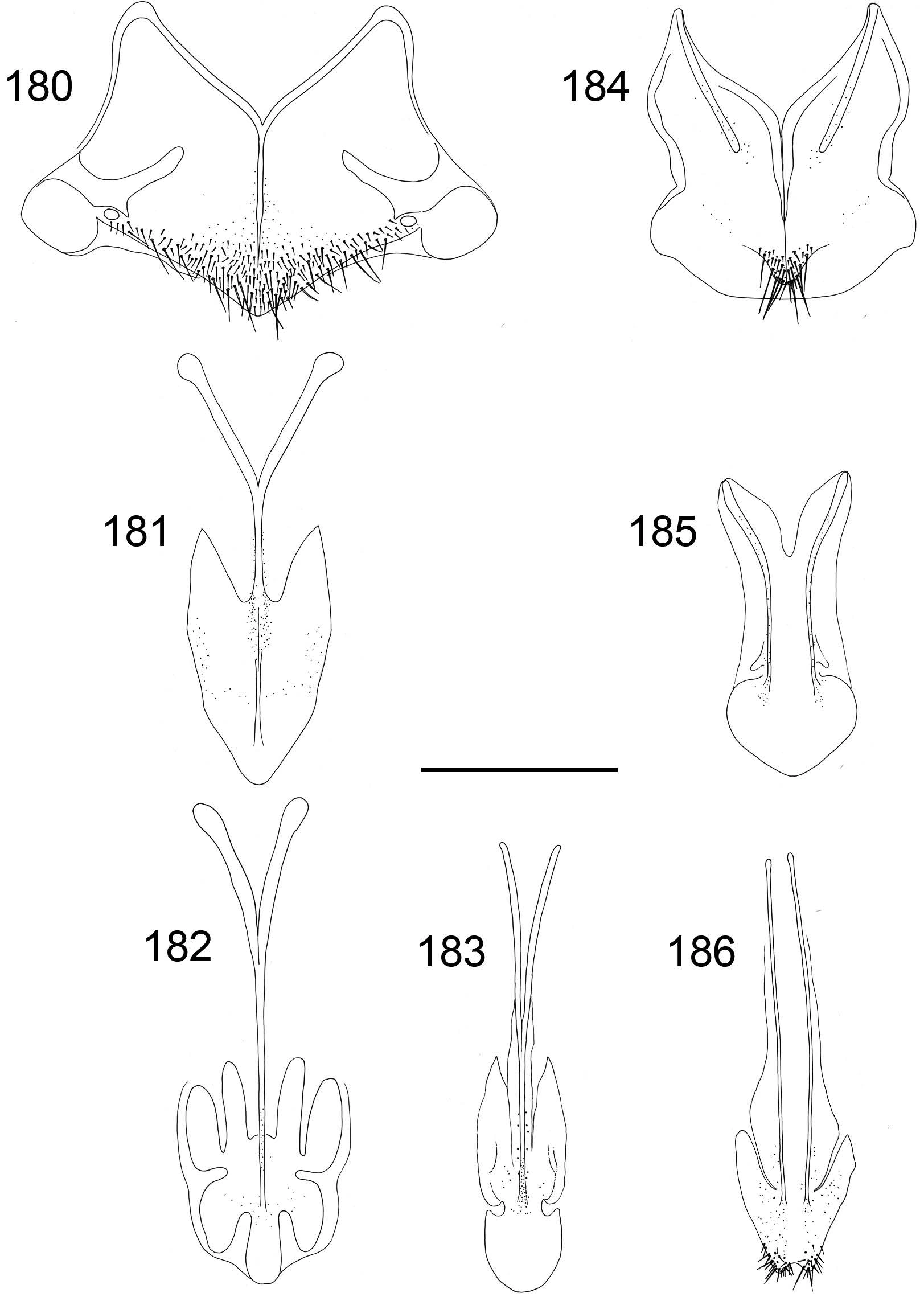
Metasoma. Length 3.8–4.9 mm, maximum width 2.4–2.9 mm. Colour of tergites golden red or reddish, T1 anteriorly often greenish (Fig. 106). T1 with strong punctation, a weakly elevated medial line with sparser punctation, and very small and scattered punctures on interstices. Punctation of T2 anteriorly regular and dense, of the same strength as on T1 (Fig. 106). Punctation becoming weaker and more scattered laterally and posteriorly. T2 with prominent elevated medial line, anteriorly narrow and posteriorly broad, flat and shiny. T3 weakly saddle-shaped, with regular, strong and dense punctation, and often with elevated midline. Punctures of T3 on average as large as posteriorly on T2. Interstices usually with prominent microsculpture, whereby surface appears dull (Fig. 106). Posterior margin of T3 with four broadly separated teeth, intervals shallow. Medial interval usually about 1.5 times wider than lateral intervals. Subapical pit row with 15–20 black or bluish pits. Subapical lateral swellings relatively strong. Pubescence silvery white. Sternite colour green or blue, occasionally with golden reflections (Fig. 124). Black spots of S2 relatively large and subrectangular, their margins often vaguely delimited (Fig. 124). Ovipositor thin, similar to C. ignita and C. impressa (Fig. 92). Internal sternites and tergites similar to C. impressa (Figs 180–186). T5 relatively narrow, about three times as long as broad, and tapering posteriorly, without lateral stigmae (Fig. 181).
Figures 180–186
Internal tergites and sternites of Chrysis borealis sp. n. ♀: 180 T4 181 T5 182 T6 183 T7 184 S4 185 S5 186 S6. Illustrations based on a paratype from Sweden (MZH_GP.92691). Scale 1 mm.
Description of male
Body length 6.7–9.0 mm, forewing length 4.7–6.0 mm (n = 15).
Head. Height 1.5–2.0 mm, width 1.9–2.4 mm, length 0.8–1.1 mm, shortest interocular distance 0.3–0.4 mm. Structure and colouration as in female, but scapal basin often slightly paler, shape of transverse frontal carina more variable, pubescence longer and mandible thicker. Sides of mandible basally slightly concave, gradually converging towards apex in lateral view. Relative lengths of P/F1/F2/F3 are 1/1.8/1.3/1.2 (Fig. 178). F1 usually 1.3–1.5 times as long as F2. F4–F10 as in female, or slightly shorter.
Mesosoma. Length 2.5–3.5 mm, width anterior to tegulae 1.7–2.4 mm. Length of pronotum medially 0.3–0.6 mm and width at anterior margin 1.5–2.1 mm. Structure as in female, but colouration usually somewhat lighter and pubescence longer. Margins of pronotum more often with golden reflections, and mesoscutum sometimes entirely blue. Mesoscutellum often medially violet, not always black, whereas mesoscutellum laterally, metanotum and propodeum violet, blue or golden green. Legs green, golden green or bluish with dark brown tibiae.
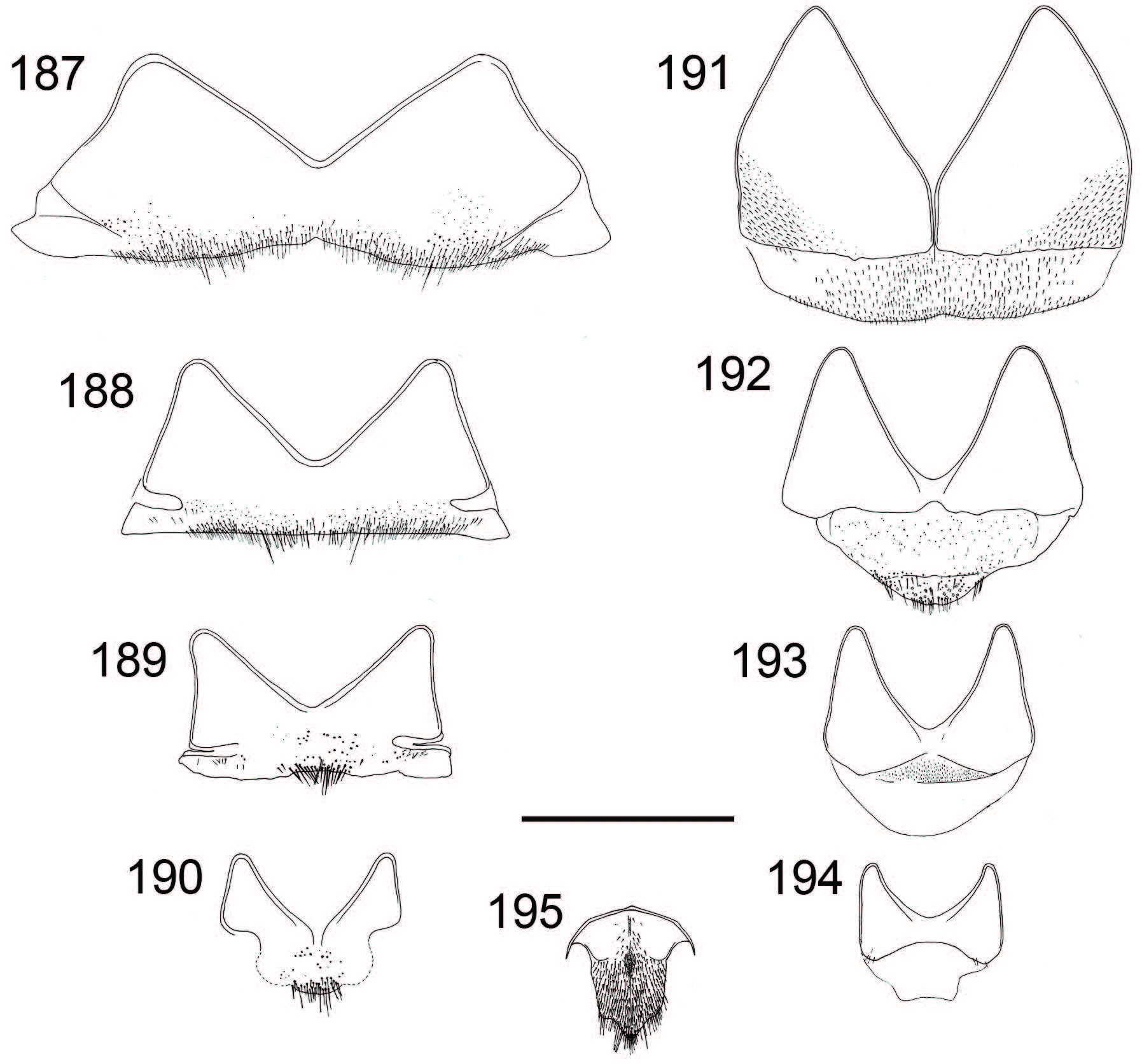
Metasoma. Length 3.3–4.4 mm, maximum width 2.1–2.8 mm. Colour of tergites as in female, but punctation of T1 and T2 usually denser and finer. T3 with very dense and homogenous punctation. Interstices shining without distinct microsculpture. T3 convex, not medially depressed as in female. Shape of apical teeth of T3 relatively variable. Medial interval narrower than or as wide as lateral intervals. Subapical pit row with 12–20 black pits. Subapical lateral swellings weak or nearly missing. Sternites with green, golden and reddish colour (Fig. 133), sometimes almost completely green. Black spots of S2 large and rounded (Fig. 133). Inner margin of paramere rounded (Fig. 136). Internal sternites and tergites similar to C. impressa (Figs 187–195). S8 about 1.2 times as long as broad, posteriorly pointed and anteriorly rounded (Fig. 195).
Figures 187–195
Internal tergites and sternites of Chrysis borealis sp. n. ♂: 187 T4 188 T5 189 T6 190 T7 191 S4 192 S5 193 S6 194 S7 195 S8. Illustrations based on a paratype from Finland (MZH_GP.92704). Scale 1 mm.
Geographic variation
Southern specimens from Estonia, Öland and Gotland are more uniform in colour than specimens from Finland, Norway and the Swedish mainland. The mesosoma of southern specimens is uniformly bright blue or violet with some greenish reflections, whereas in northern specimens, the mesoscutum and central part of the pronotum are commonly black or dark violet, and the margins of the pronotum and mesoscutellum are, in contrast, greenish or even golden green, especially in the males.
DNA-analysis
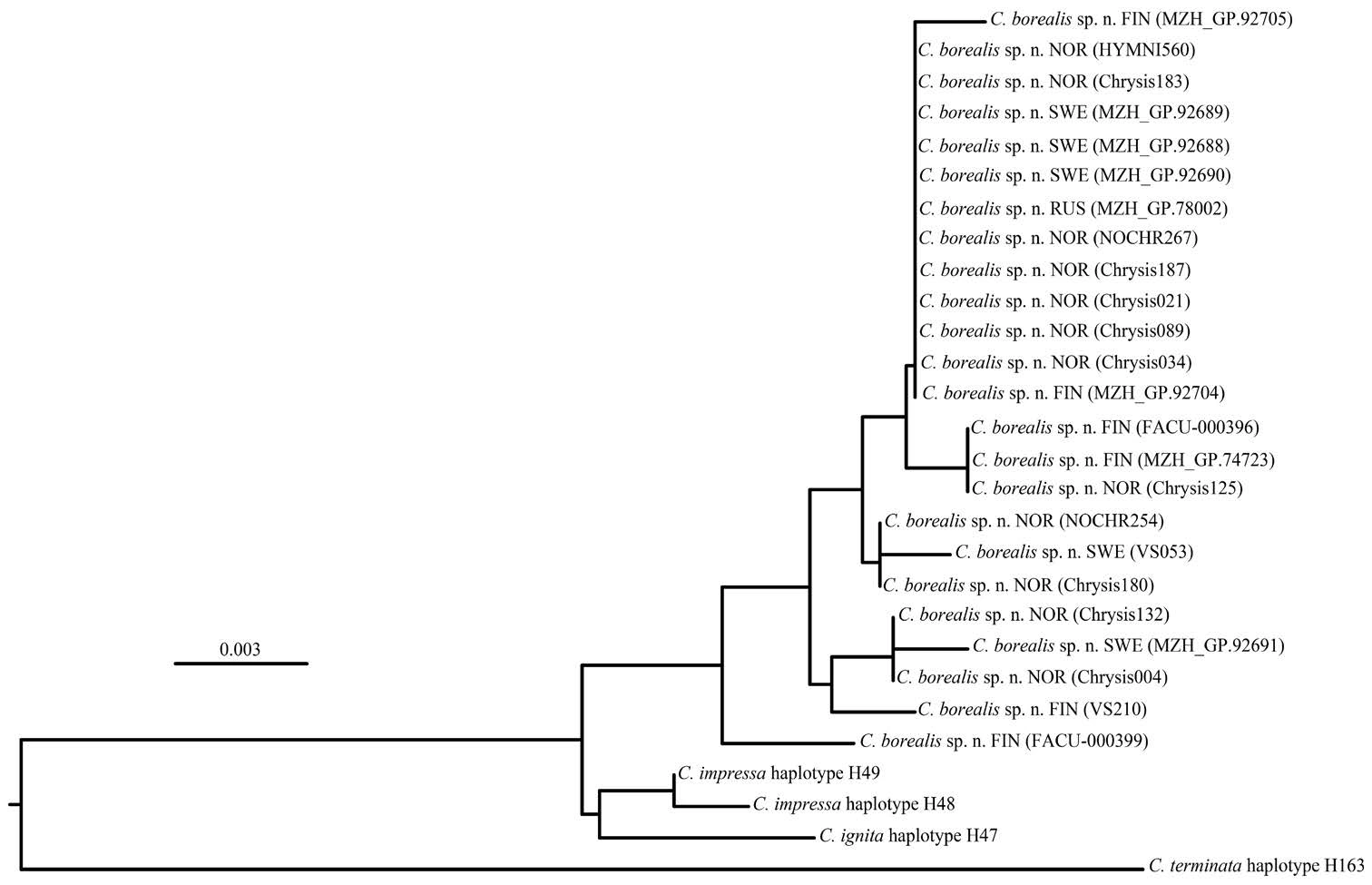
Variable positions of the DNA barcode sequences of C. borealis sp. n. and its sibling species, C. ignita and C. impressa, are presented in Table 2. Despite relatively high intraspecific variability, there are two diagnostic nucleotide mutations conserved in all sequences of C. borealis sp. n. compared to C. ignita and C. impressa: G instead of A in position 241 and T instead of C in position 340. Additionally, there are three transitions shared with C. impressa, but differing from C. ignita, and one transition shared with C. ignita, but differing from both haplotypes of C. impressa. All samples of C. borealis sp. n. cluster together indicating their closer relationship with each-other than the other two species (Fig. 196).
Distribution
Denmark, Estonia, Finland, Norway, Sweden. Rare.
West Palearctic (?), general distribution poorly known. So far only known from the Nordic and Baltic countries and north-western Russia (Leningrad Oblast, Republic of Karelia, Murmansk Oblast) (Paukkunen et al. 2014).
Biology
Habitat: rocky outcrops, cliffs, alpine meadows, forest margins. Often found on islands of the Baltic Sea and in Lapland, where other species of the C. ignita group are uncommon. Adults have been found sitting on sun-exposed leaves of Tussilago and flowers of Apiaceae. They have also been collected using yellow pan traps.
Flight period: late May to late August. A few specimens have been collected in early May and September.
Host: Ancistrocerus parietum (Linnaeus) (Vespidae), based on records of C. borealis sp. n. from islands and other coastal localities, where A. parietum is the only species of Eumeninae. In northern alpine areas, where A. parietum is not present, A. scoticus (Curtis) is the most likely host species.
Etymology
The species epithet borealis is a Latin word derived from the Greek boreas which means north. We use it here as an adjective in the feminine case. The interpretation of borealis should be “northern”.
Remarks
C. borealis sp. n. is very closely related to C. impressa, and cannot always be determined with certainty by morphological characters only. It is also easily confused with C. schencki. The colouration of C. impressa can sometimes be relatively dark and similar to C. borealis n. sp., which possibly could be caused by cool weather during the larval and pupal development. Generally, the colouration of chrysidids becomes darker in northern and alpine localities with cool climatic conditions. C. borealis n. sp. is mainly found in cooler habitats than C. impressa, so it could be claimed to constitute only a dark ecological form of C. impressa and not a distinct species. The slight, but constant divergence in the DNA barcode sequence and the statistically significant difference in the F1/F2 ratio, however, supports the treatment of C. impressa and C. borealis n. sp. as distinct, but evolutionarily young sibling species. It is also noteworthy that the DNA barcode sequence of C. ignita is very similar to C. borealis n. sp. and C. impressa, but due to significant morphological and ecological differences C. ignita undoubtedly forms a distinct species. Previous studies indicate that morphological and molecular differences between biologically welldefined species can be extremely small or even nonexistent in the C. ignita group (Soon et al. 2014). An example is provided by C. mediata and C. solida, which are not always reliably separable by morphological characters or DNA barcodes, but represent ecologically well separated species with different hosts and habitats (Soon et al. 2014). Several authors have earlier identified specimens of C. borealis sp. n. erroneously as C. mediadentata Linsenmaier, 1951 (Paukkunen et al. 2014). For example, Erlandsson (1971) reported C. mediadentata from Sweden, Norway and eastern Fennoscandia based on such misidentified specimens. The dark colouration of C. mediadentata can indeed resemble that of C. borealis sp. n., but the species is easily distinguished by the breadth of the ovipositor, which in C. borealis sp. n. is of the thin ignita-type (as in Fig. 92), and in C. mediadentata of the thick solida-type (as in Fig. 91) (Linsenmaier 1987). Additionally, the apical teeth of C. mediadentata have deeper intervals, and the two central teeth are more narrowly separated. The mandible of C. mediadentata is thicker and the inner margin of the paramere of the male genitalia is angulate (as in Fig. 137). So far C. mediadentata has not been found in the Nordic and Baltic countries. The northernmost confirmed records are from northern Germany (Jacobs and Kornmilch 2007).