Limnozetes foveolatus
Limnozetes foveolatus is the smallest representative of the genus Limnozetes. It is aquatic, requires acidic water, and is restricted to Sphagnum-dominated peatlands. Like all Limnozetes species, it reproduces parthenogenetically, that is, females are produced from unfertilized eggs.
- Innhold
- Description
- Look-alikes
- Biology
- Ecology
Description
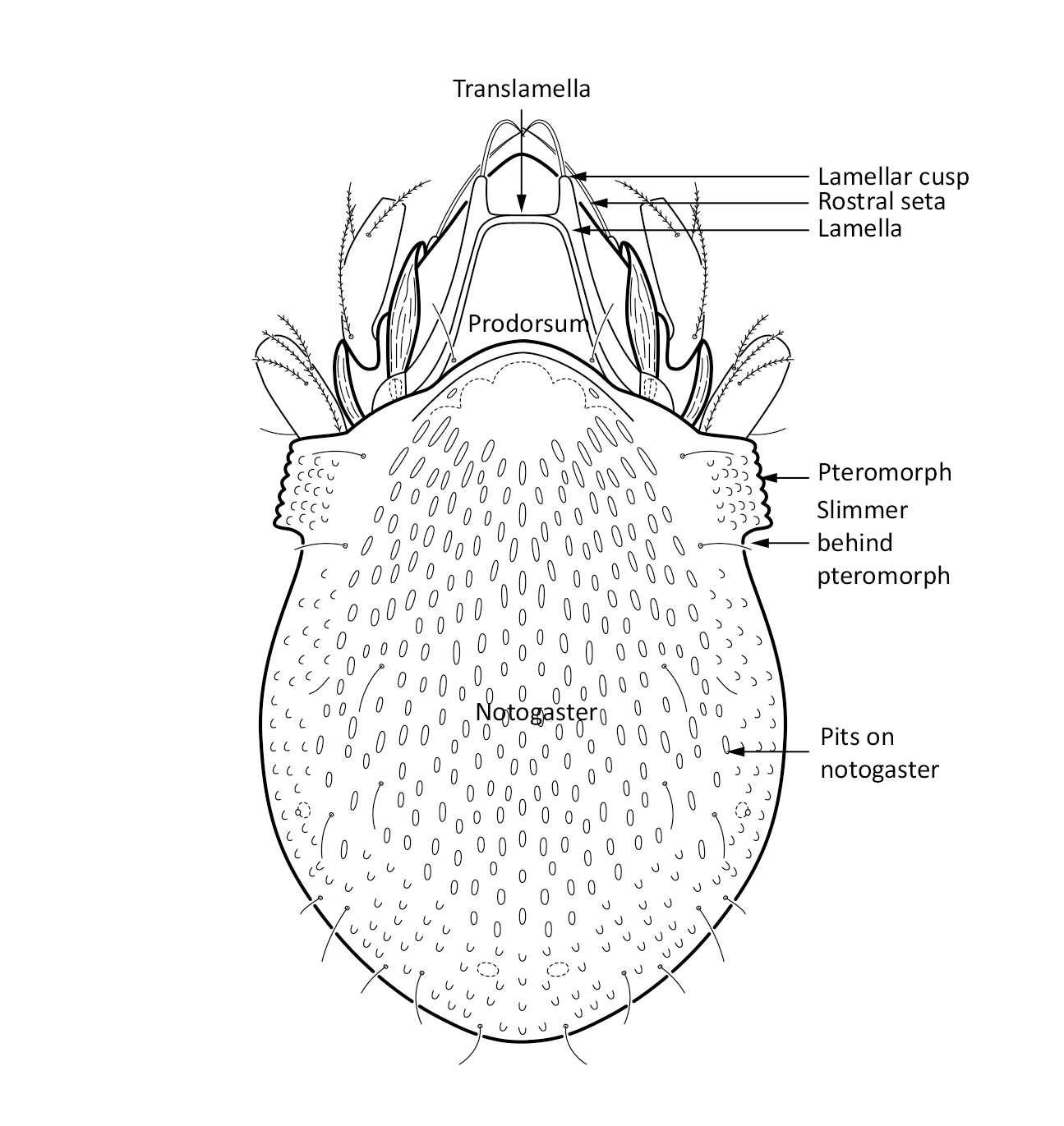
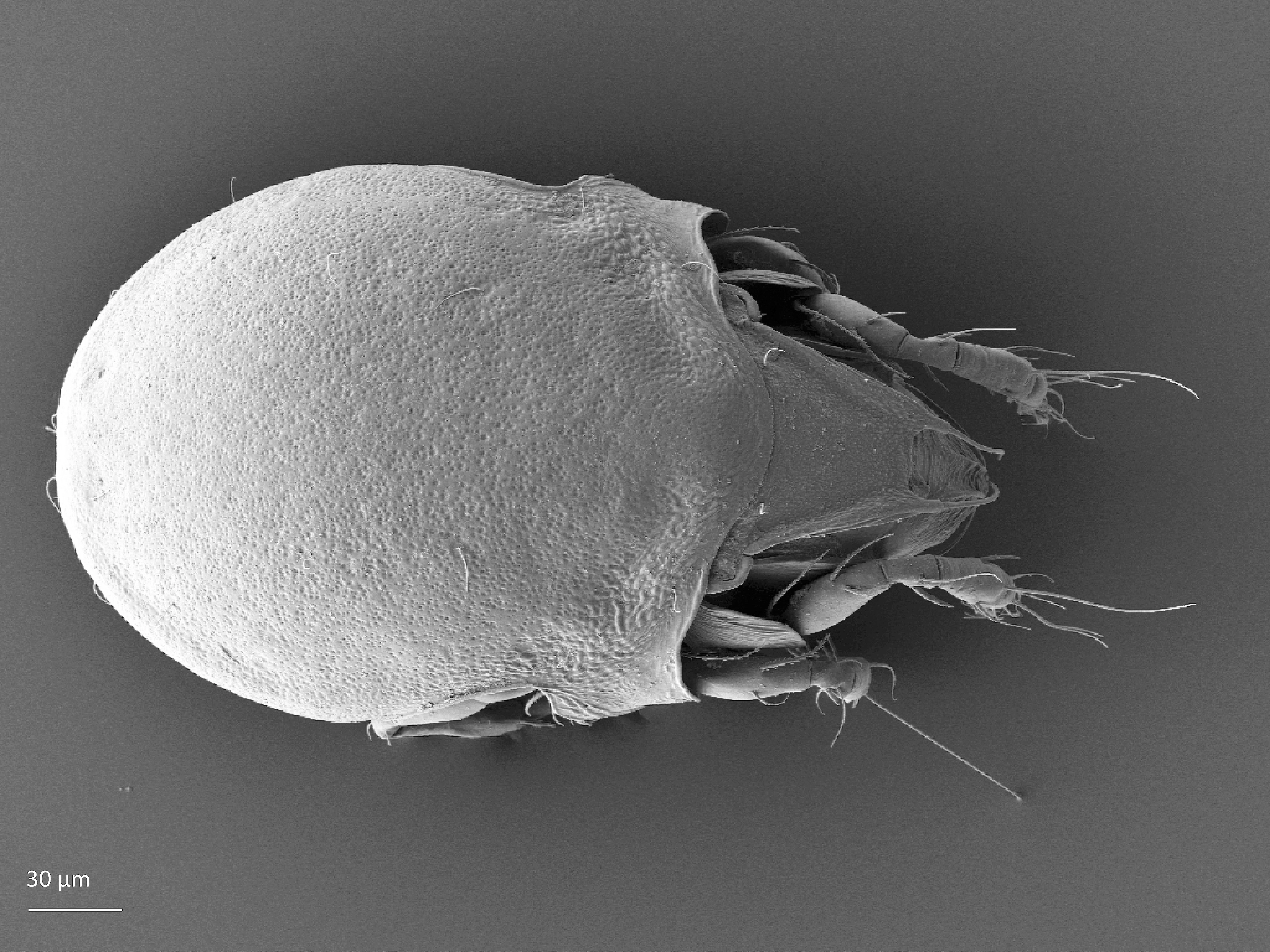
Adult: The length of the body ranges between 266–292 µm, on average 287 µm, and the width is on average 168 µm. There is a pair of pteromorphs, that is wing-like structures, in the front of the notogaster, and the body is slimmer behind the pteromorphs (marked in Fig. 1). The notogaster surface has characteristic pits. The prodorsum has a pair of lamellae, which are connected by a thin translamella. Each of the lamella has a cusp that is rounded in the front. The bothridial seta is absent. Among the prodorsal setae, the longest is the rostral seta (ro). The notogaster has 10 pairs of short and smooth setae (Seniczak and Seniczak 2009).
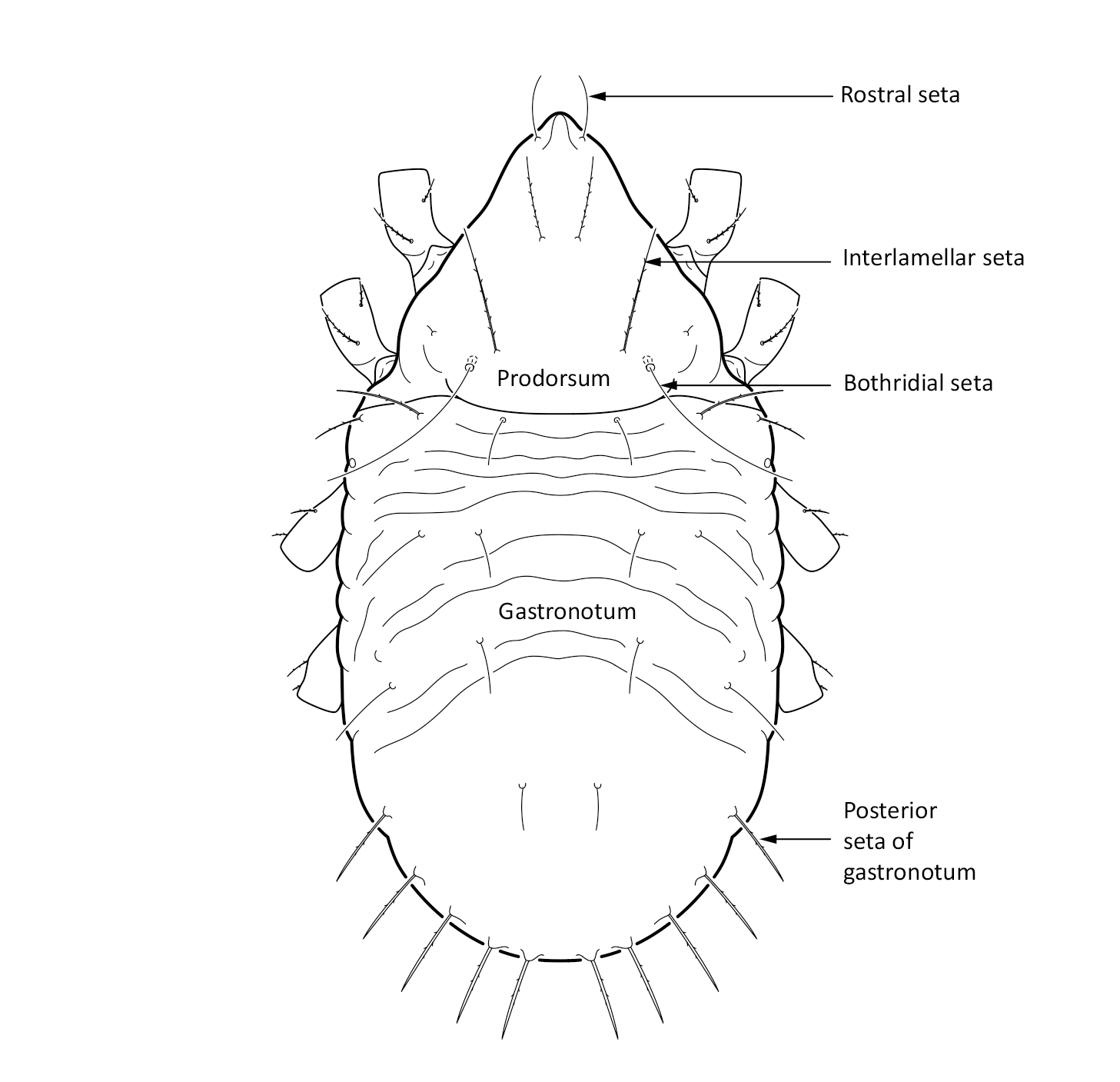
Juvenile stages: Larva: body length 171 µm, width 91 µm. Protonymph: body length 198 µm, width 106 µm. Deutonymph: body length 238 µm, width 122 µm. Tritonymph: body length 277 µm, width 142 µm. The color is white. The bothridial seta is long and setiform (Fig. 2). The gastronotum, that is the main body, has wrinkled cuticle. The posterior setae on the gastronotum are thicker than other setae (Seniczak and Seniczak 2009, described as Limnozetes palmerae Behan-Pelletier, 1989).
Fig. 1. Dorsal view of Limnozetes foveolatus, adult.
Fig. 2. Dorsal view of Limnozetes foveolatus, juvenile (tritonymph).
Look-alikes
Limnozetes foveolatus is similar to Limnozetes ciliatus and was originally described by Willmann (1939) as a subspecies of Limnozetes ciliatus (Schrank, 1803). However, Limnozetes foveolatus is smaller and slimmer: length/width ratio of the adult is 1.7 in Limnozetes foveolatus vs 1.6 in Limnozetes ciliatus. The notogaster of the adult Limnozetes foveolatus has characteristic pits, while in Limnozetes ciliatus it is smooth and finely punctuated. The juveniles of Limnozetes foveolatus have an interlamellar seta on the prodorsum which is more than two times longer than the rostral seta, while in Limnozetes ciliatus both these setae are of a similar length. In Limnozetes foveolatus only the posterior setae on the notogaster are thicker, while in Limnozetes ciliatus all peripheral setae on the notogaster, not only the posterior ones, are thick, stiff, and rather long (Seniczak and Seniczak 2009).
Biology
The reproduction is parthenogenetic, meaning that only females are present, and they are produced from unfertilized eggs. Its feeding preferences and time of development are unknown.
Ecology
This species occurs in peatlands but seems to have narrower ecological tolerance than Limnozetes ciliatus. It is less common than Limnozetes ciliatus, but often co-occurs with it, and usually outnumbers it in dripping-wet habitats. At the edges of peatland ponds and pools it can make over 90% of the oribatid mites (Seniczak 2011). Limnozetes foveolatus reacts quickly to seasonal changes, while the abundance of Limnozetes ciliatus remains similar throughout the year (Seniczak et al. 2019). It is restricted to dystrophic water bodies, with water having dark color, low pH, low content of calcium and high content of organic carbon (Seniczak et al. 2022). Limnozetes foveolatus is the most abundant oribatid species in dystrophic ponds and pools and makes 56–93% of all Oribatida there (Seniczak 2011). It is a good bioindicator of the peatland conditions; its abundance is low in disturbed peatlands (Seniczak et al. 2016). However, it quickly colonizes restored peatlands if water parameters resemble those of dystrophic water bodies (Seniczak et al. 2022).
Distribution
Limnozetes foveolatus is considered a Holarctic species (Subías 2004), meaning that it is found in the northern continents of the world.
Habitat
The species lives in Sphagnum-dominated peatlands and is confined to dystrophic water bodies.
Findings in Norway
It was first reported in 2020 from peatlands in Vestland (Seniczak et al. 2020).
References
Seniczak A (2011). Mites (Acari) of the Shores of Forest Lakes and Ponds in Northern Poland, With Species Analysis of Oribatida. Wydawnictwa UTP, Bydgoszcz, 1–231.
Seniczak A, Seniczak S, Graczyk R, Waldon-Rudzionek B, Nowicka A and Pacek S (2019). Seasonal dynamics of oribatid mites (Acari, Oribatida) in a bog in Poland. Wetlands, 39(6), 853–864. doi.org/10.1007/s13157-019-01125-2
Seniczak A, Seniczak S, Iturrondobeitia JC, Gwiazdowicz DJ, Waldon-Rudzionek B, Flatberg KI and Bolger T (2022) Mites (Oribatida and Mesostigmata) and vegetation as complementary bioindicators in peatlands. Science of the Total Environment 851(2), 158335. doi.org/10.1016/j.scitotenv.2022.158335
Seniczak A, Seniczak S, Iturrondobeitia JC, Solhøy T and Flatberg KI (2020) Diverse Sphagnum mosses support rich moss mite communities (Acari, Oribatida) in mires of western Norway, Wetlands, 40, 1339–1351. doi.org/10.1007/s13157-019-01236-w
Seniczak A, Seniczak S, Maraun M, Graczyk R and Mistrzak M (2016). Oribatid mite species numbers increase, densities decline and parthenogenetic species suffer during bog degradation. Experimental and Applied Acarology 68, 409–428. doi.org/10.1007/s10493-016-0015-8
Seniczak S and Seniczak A (2009). Morphology of some species of Limnozetes Hull, 1916 (Acari: Oribatida: Limnozetidae), and keys to the larvae and nymphs. Annales Zoologici, 59(3), 387–396. doi.org/10.3161/000345409X476459
Subías LS (2004). Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (excepto fósiles). Graellsia 60 (número extraordinario), 3–305.
Willmann C (1939). Die Moorfauna des Glatzer Schneeberges. 3. Die Milben der Schneebergmoore. Beiträge zur Biologie des Glatzer Schneeberg (Breslau) 5, 427–458.