Limnozetes ciliatus
Limnozetes ciliatus is one of the most common and abundant oribatid species found in peatlands. Like all Limnozetes species it is aquatic, it reproduces and lives in water or at its margin and occurs only in acidic water. It is found in submerged peatland microhabitats, like hollows, pools, and ponds. It indicates good water conditions in the peatland; if the peatland gets dried out, this species disappears quickly.
- Innhold
- Description
- Look-alikes
- Biology
- Ecology
Description
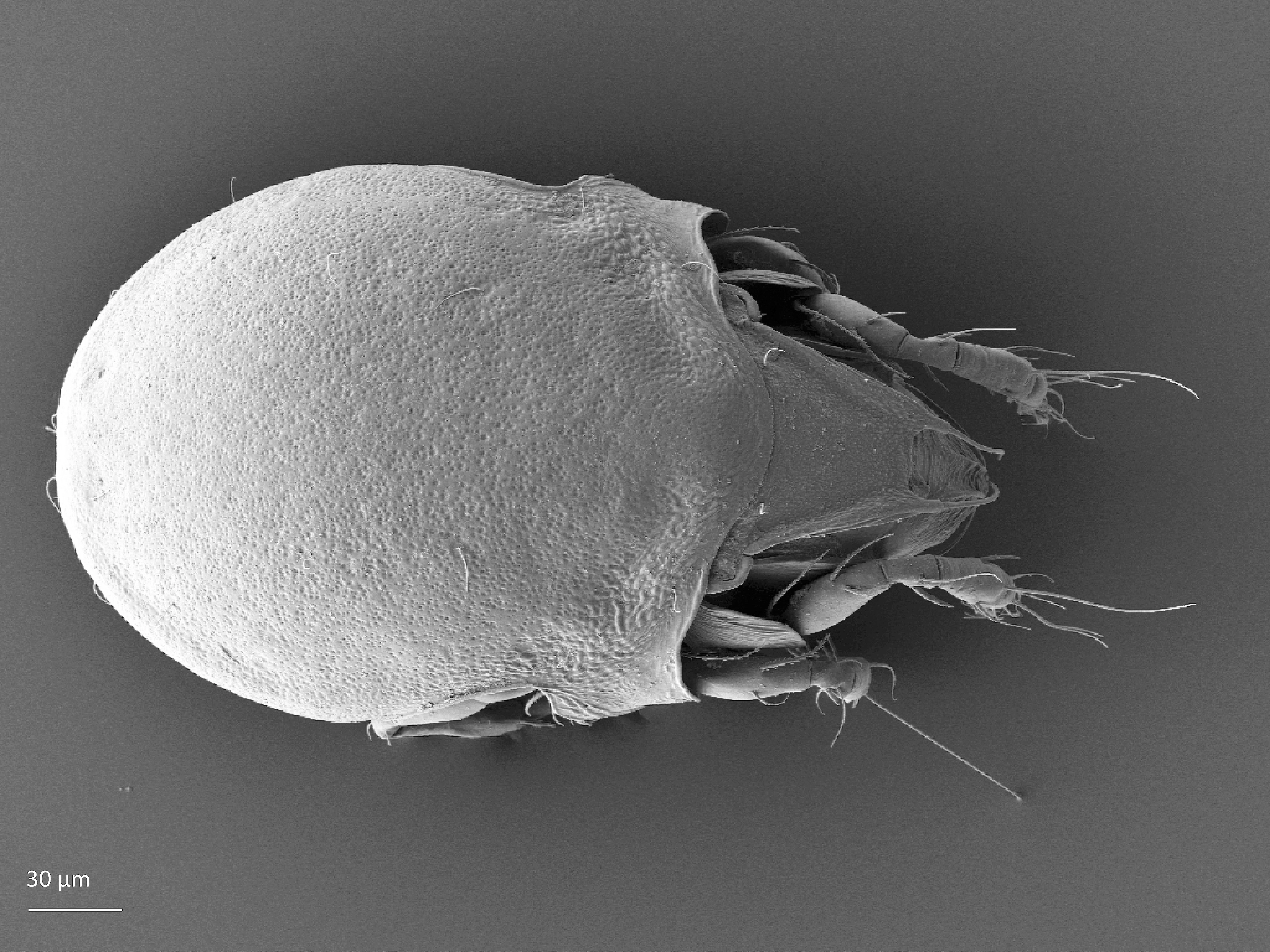
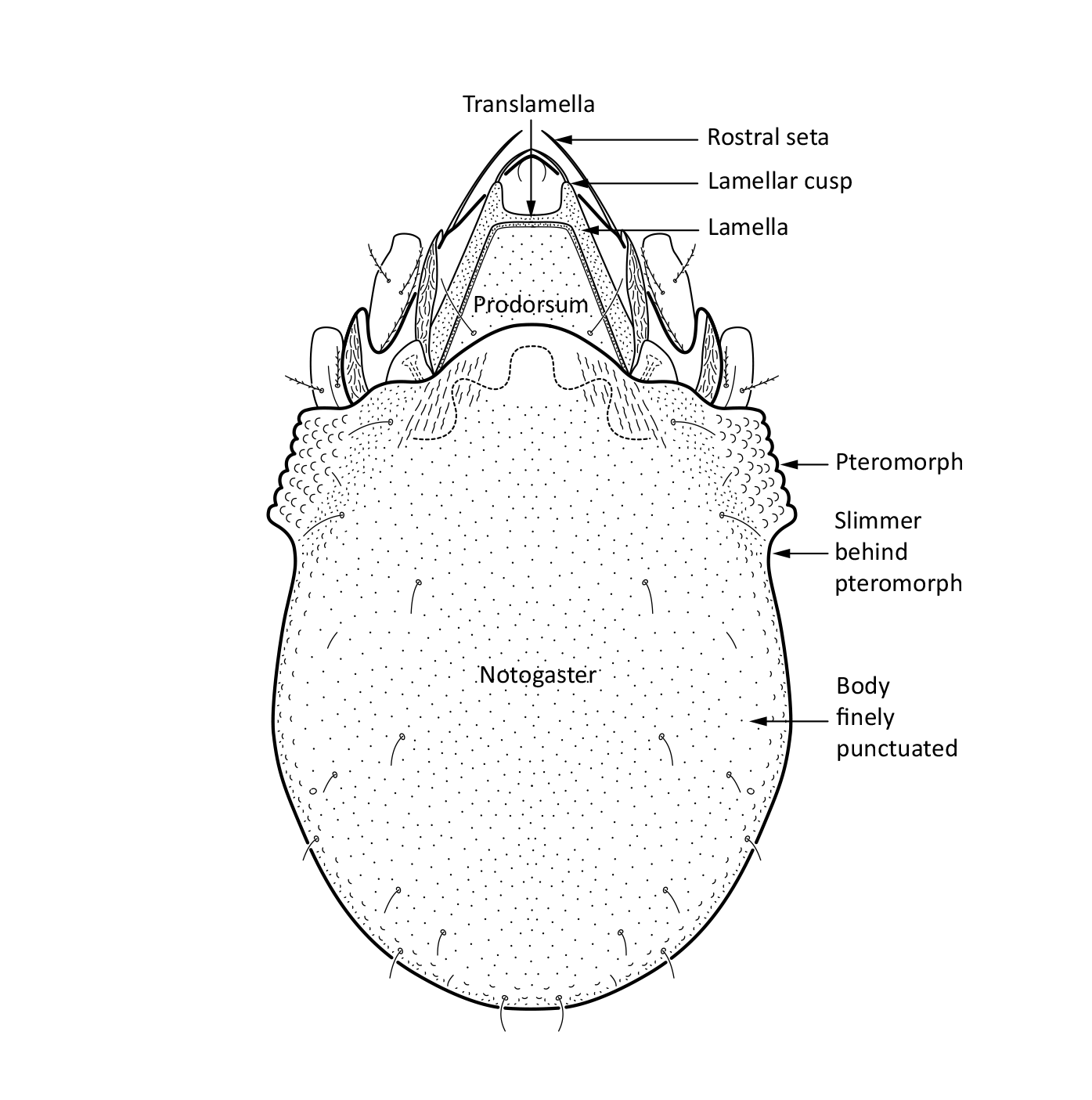
Adult: The length of the body is on average 330 µm and the width is 204 µm. The body has oval shape and brown color. There is a pair of pteromorphs, that is, wing-like structures, in the front of the notogaster, and the body is slimmer behind the pteromorphs (marked in Fig. 1). The body surface is finely punctuated. The prodorsum has a pair of lamellae, which are connected by a translamella. Each of the lamellae has a cusp that is rounded in the front. The bothridial seta is usually absent but sometimes it is setiform or globose. Among prodorsal setae, the longest is the rostral seta (ro). The notogaster has 10 pairs of short and smooth setae (Fig. 1) (Weigmann 2006, Seniczak and Seniczak 2009).
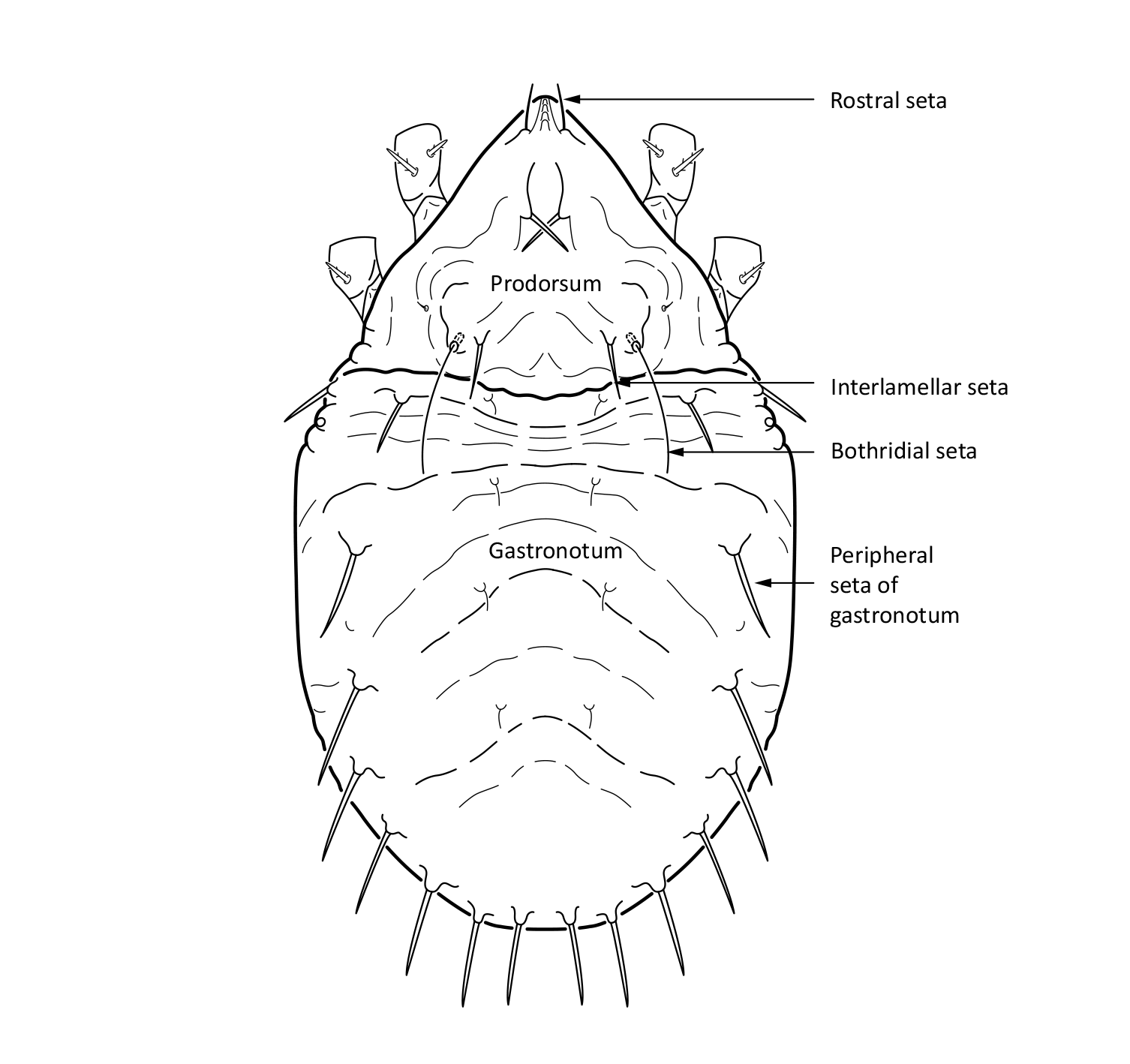
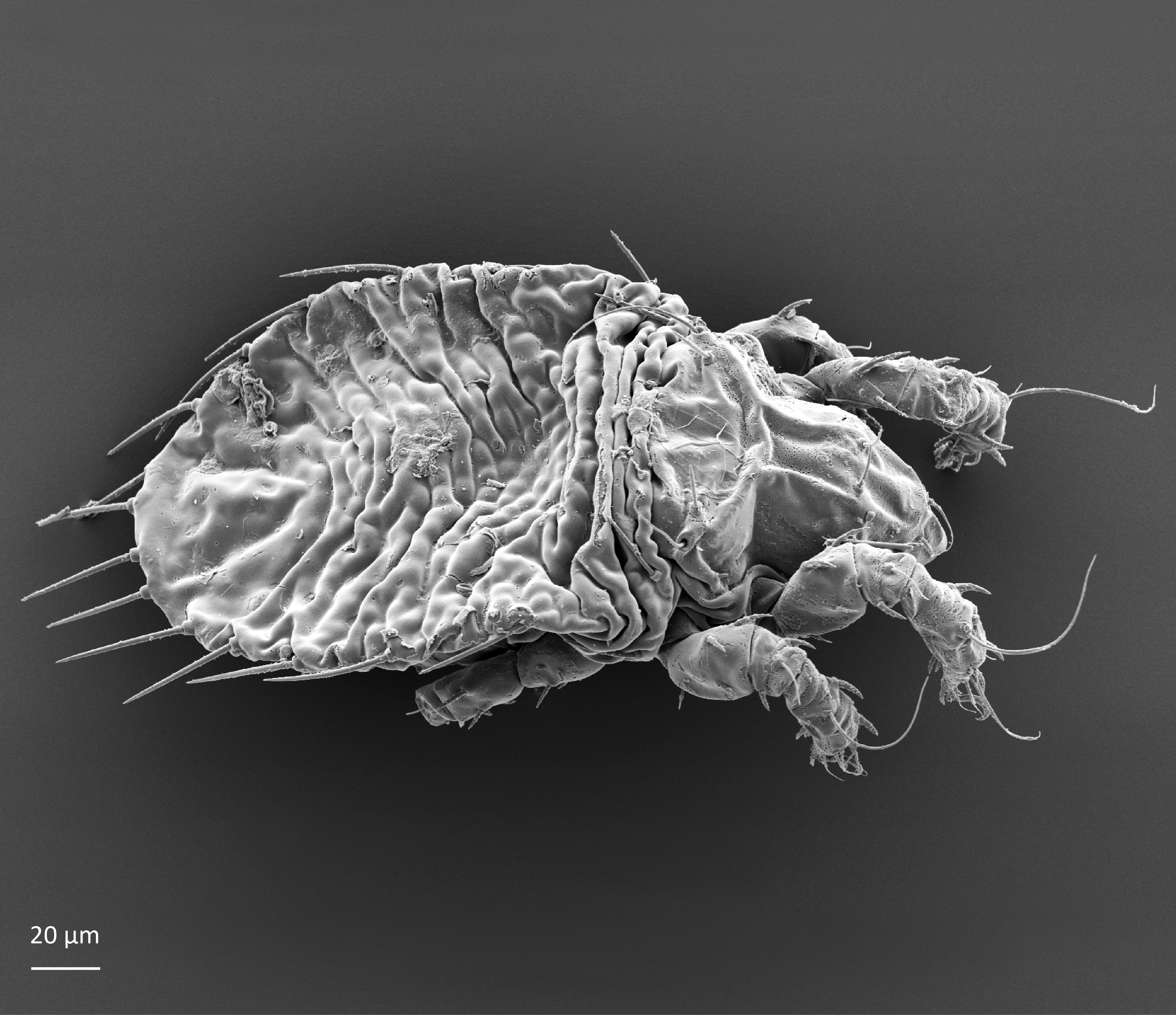
Juvenile stages: Larva: body length 185 µm, width 109 µm. Protonymph: body length 224 µm, width 128 µm. Deutonymph: body length 274 µm, width 150 µm. Tritonymph: body length 297 µm, width 168 µm. The color is white. The bothridial setae is long and setiform (Fig. 2). The gastronotum, that is the main body, has wrinkled cuticle (Figs 2 and 3). The peripheral setae on the gastronotum are thick, stiff, and rather long, while other gastronotal setae are thin and short (Seniczak and Seniczak 2009).
Fig. 1. Dorsal view of Limnozetes ciliatus, adult.
Fig. 2. Dorsal view of Limnozetes ciliatus, juvenile (tritonymph).
Look-alikes
Limnozetes ciliatus is similar to Limnozetes foveolatus Willmann, 1939, both as adult and juvenile forms. Adult Limnozetes ciliatus is larger by ca 15% compared to Limnozetes foveolatus. It is wider: length/width ratio is 1.6 in Limnozetes ciliatus while it is 1.7 in Limnozetes foveolatus. In Limnozetes ciliatus the notogaster is finely punctuated, while in Limnozetes foveolatus it has characteristic microtubercles. The juveniles of Limnozetes ciliatus are larger than those of Limnozetes foveolatus. In Limnozetes ciliatus the prodorsal interlamellar seta and rostral seta are of a similar length, while in Limnozetes foveolatus the interlamellar seta is more than two times longer than the rostral seta. In Limnozetes ciliatus all peripheral setae on the gastronotum are thick, stiff, and rather long, while in Limnozetes foveolatus only the posterior setae on the gastronotum are thicker.
Biology
The reproduction is parthenogenetic, only females are present, and they are produced from unfertilized eggs. At a temperature of 25°C the species had the highest fertility, and the females laid eggs at intervals of 4–31 days, one egg at a time, with a maximum of 11 eggs. Developmental time from egg to adult is 94 days (Kuriki 2008). Limnozetes ciliatus has wide feeding preferences, and eats fungi, dead plant matter, conifer pollen, and sporadically arthropod fragments (Behan-Pelletier and Hill 1983, Lehmitz and Maraun 2016).
Fig. 3. Dorsolateral view of Limnozetes ciliatus, juvenile.
Ecology
Under laboratory conditions, its metabolism and fecundity were highest at 25°C. However, its development was fastest at 20°C (78 days), longer at 25°C (93.6 days) and the longest at 15°C (142.2 days). Adults collected from the field showed seasonal differences in fecundity, which was highest in summer and lowest in autumn (Kuriki 2008). It has a wide tolerance towards variations in temperature and does not migrate due to diurnal changes in the temperature (Popp 1962).
Its diet varies depending on the season. When feeding on grasses, it deposited up to 1,528 fecal pellets during its whole life, which documents the important role this species has in the decomposition of organic matter (Kuriki 2008). The species is often eaten by odonate naiads (Behan-Pelletier and Bisset 1994).
Distribution
Limnozetes ciliatus is considered to be a Holarctic species (Subías 2004), which means that it is found in the northern continents of the world.
Habitat
The species lives in Sphagnum-dominated peatlands and is confined to aquatic, acidic microhabitats (Behan-Pelletier and Bisset 1994, Schatz and Behan-Pelletier 2007). It is the most common representative of the genus, and it often occurs abundantly. For example, at the pond edge at Fløyen Mtn. in Bergen, it had the density of 16 thousand specimens per m² and made up 30% of all oribatid mites (Seniczak et al. 2010). When the peatland gets dried out, the species gets replaced by generalists, e.g., Tectocepheus velatus (Michael, 1880) and Oppiella nova (Oudemans, 1902) (Markkula 1986).
Findings in Norway
The species was first reported in 1929 from a peatland on Herdla island (Vestland), where as many as 700 specimens were found in two moss samples (Willmann 1929). Sig Thor (1937) recorded only one specimen from Årkvisla in Lier (Viken), but he probably studied other microhabitats than a wet peatland. In mires, this species occurs abundantly and was found in different locations in Norway, in Vestland (Solhøy 1979, Seniczak et al. 2010, 2020), Trøndelag (A. Seniczak, pers. comm. 2023) and Nordland (Karppinen 1971). It was also reported in fossil samples from Holocene lake sediments in Norway, at Kråkenes (Solhøy and Solhøy 2000) and Trettetjørn (Riva-Caballero 2003, Larsen et al. 2006, Riva-Caballero et al. 2010).
References
Behan-Pelletier VM and Bisset B (1994). Oribatida of Canadian peatlands. Memoirs of the Entomological Society of Canada, 126(169), 73–88. doi.org/10.4039/entm126169073-1
Behan-Pelletier VM and Hill SB (1983). Feeding habits of sixteen species of Oribatei (Acari) from an acid peat bog, Glenamoy, Ireland. Revue d’Écologie et Biologie du Sol, 20(2), 221–267.
Karppinen E (1971). Studies on the Oribatei (Acari) of Norway. Annales Entomologici Fennici, 37, 30–53.
Kuriki G (2008). The life cycle of Limnozetes ciliatus (Schrank, 1803) (Acari: Oribatida). Journal of the Acarological Society of Japan, 17(2), 75-85.
Larsen J, Bjune AE and Riva-Cabellero A (2006). Holocene environmental and climate history of Trettetjørn, a low-alpine lake in western Norway, based on subfossil pollen, diatoms, oribatid mites and plant macrofossils. Arctic, Antarctic, and Alpine Research,38, 571–583. doi.org/10.1657/1523-0430(2006)38[571:HEACHO]2.0.CO;2
Lehmitz R and Maraun M (2016). Small-scale spatial heterogeneity of stable isotopes signatures (δ15N, δ13C) in Sphagnum sp. transfers to all trophic levels in oribatid mites. Soil Biology and Biochemistry, 100 (1), 242–251. doi.org/10.1016/j.soilbio.2016.06.005.
Markkula I (1986). Comparison of the communities of oribatids (Acari: Cryptostigmata) of virgin and forest ameliorated pine bogs. Annales Zoologici Fennici, 23, 33–38.
Popp E (1962). Semiaquatile Lebensräume (Bülten) in Hoch- und Niedermooren. 2 Teil. Die Milbenfauna. Internationale Revue der gesamten Hydrobiologie, 47(4), 533–579.
Riva-Caballero A (2003). Oribatid mite communities in bogs along an altitudinal gradient and palaeoecological reconstruction using fossil oribatid mites in Western Norway. Unpublished M.Sc. thesis, University of Bergen, Norway, 1–67.
Riva-Caballero A, Birks HJB, Bjune AE, Birks HH and Solhøy T (2010). Oribatid mite assemblages across the tree-line in western Norway and their representation in lake sediments. Journal of Paleolimnology, 44, 361–374. doi.org/10.1007/s10933-010-9411-y
Schatz H and Behan-Pelletier V (2007). Global diversity of oribatids (Oribatida: Acari: Arachnida). In: Balian EV, Lévêque C, Segers H and Martens K (eds) Freshwater Animal Diversity Assessment. Developments in Hydrobiology, vol 198. Springer, Dordrecht. Doi.org/10.1007/978-1-4020-8259-7_35
Seniczak A, Seniczak S, Iturrondobeitia JC, Solhøy T and Flatberg KI (2020). Diverse Sphagnum mosses support rich moss mite communities (Acari, Oribatida) in mires of western Norway, Wetlands, 40, 1339–1351. doi.org/10.1007/s13157-019-01236-w
Seniczak A, Solhøy T, Seniczak S and Riva-Caballero A (2010). Species composition and abundance of the oribatid fauna (Acari, Oribatida) at two lakes in the Fløyen area, Bergen, Norway. Biological Letters, 47(1), 11–19. doi.org/10.2478/v10120-009-0014-0
Seniczak S and Seniczak A (2009). Morphology of some species of Limnozetes Hull, 1916 (Acari: Oribatida: Limnozetidae), and keys to the larvae and nymphs. Annales Zoologici, 59(3), 387–396. doi.org/10.3161/000345409X476459
Solhøy IW and Solhøy T (2000). The fossil oribatid mite fauna (Acari, Oribatida) in late glacial and early Holocene sediments in Kråkenes Lake, Western Norway. Journal of Paleolimnology, 23, 35–47. doi.org/10.1023/A:1008068915118
Solhøy T (1979). Oribatids (Acari) from an oligotrophic mire in western Norway. Fauna Norvegica Ser B 26, 91–94.
Subías LS (2004). Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (excepto fósiles). Graellsia 60 (número extraordinario), 3–305.
Thor S (1937). Übersicht der norwegischen Cryptostigmata mit einzelnen Neben bemerkungen. Nyt Magazin for Naturvidenskeberne, 77, 275–307.
Weigmann G (2006). Hornmilben (Oribatida). Die Tierwelt Deutschlands. 520 pp. Vol. 76, Goecke and Evers, Keltern.
Willmann C (1929). Oribatiden von der Insel Herdla. Bergens Museums Årbok 1929, Naturvidenskap 5, 1–6.